A powerful natural supplement to support the immune system
AHCC®+3 is a supplement containing only natural ingredients to provide immune support to those who need it. It is:
- made in UK, based on authentic AHCC® sourced from the AminoUp Corporation in Japan;
- complemented with 3 additional immune boosters: zinc, selenium and vitamin C, which work together to boost both the immunity you are born with and the immunity you develop over time in response to bacteria and viruses; and,
- especially designed for people:
- fighting both acute and chronic infections (e.g. flu, respiratory syncytial virus, coronavirus, Dengue fever, HPV, herpes simplex, Varicella-Zoster virus) and their long-term effects (e.g. long Covid),
- who have undergone cancer treatment; or
- whose immunity is weakened or disturbed.
Lactose free, gluten free, GMO free, hormone free and 100% vegan.
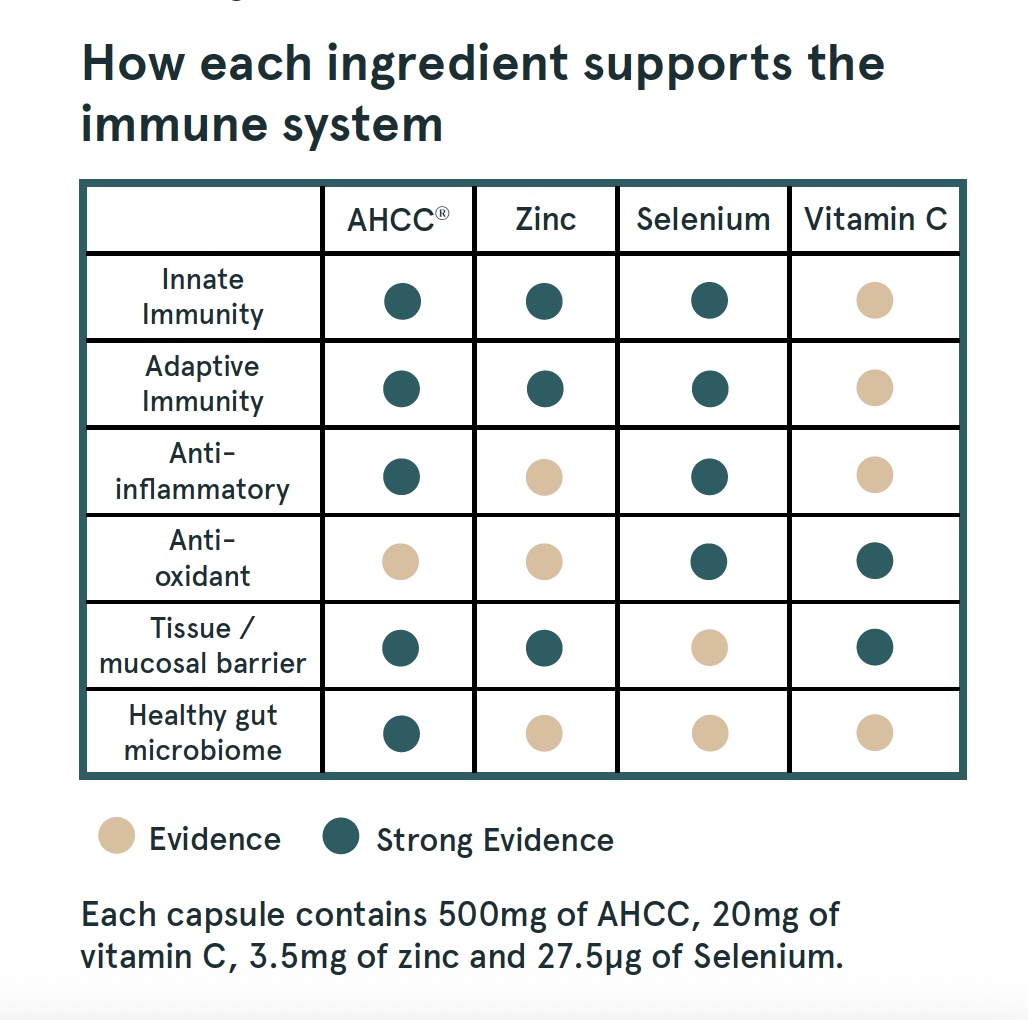
AHCC® (cultured shiitake extract): used to modulate immunity in more than 40 countries for over 30 years.
Zinc: vital for the development and activation of immune cells; can regulate the body’s inflammatory response to infection and shorten the time an infection lasts.
Selenium: supports the production of antibodies and the protective activity of white blood cells; acts as an antioxidant, protecting the body from free radicals (unstable molecules that can damage cells); helps regulate excessive immune response that can cause chronic inflammation.
Vitamin C: an antioxidant, which supports the immune system by protecting cells from free radical damage and enhancing the body’s natural defences.
Each bottle contains 60 Capsules (30 Servings)
Each capsule contain 500mg of AHCC®, 20mg of vitamin C, 3.5mg of zinc and 27.5μg of Selenium
Other ingredients:
Vegan capsule (HPMC), brown rice flour, silicon dioxide.
Efficacy and safety of AHCC® has been proven in over 100 scientific publications including more than 30 human clinical studies.
Clinical dosing (the dosing that is used in clinical trials for higher efficacy)
- Take six capsules daily; two after each meal or in six-hourly intervals
- Continue taking for three to six months.
- After the course, seek a doctor’s advice for your next steps. You can continue the clinical dosing, step down to maintenance dosing or stop taking AHCC®, depending on your condition.
Maintenance dosing
- Take two capsules daily. It is recommended to take AHCC® on an empty stomach, though it can be
taken with food if preferred. - Continue taking for at least three months for full benefits, though you may feel some results
immediately. - You can take AHCC® as long as you need.
- The clinical dose is that used in clinical trials to achieve the results they report (see evidence in the FAQs).
- The standard dose is for general health maintenance and step down after 3 to 6 months of clinical dosing.
AHCC®+3
AHCC+3 is a natural supplement to support the immune system. Made in the UK, it is based on authentic AHCC sourced from Japan, with 3 additional immune boosters: zinc, selenium and vitamin C. It is recommended for people fighting infections (e.g. flu, respiratory syncytial virus, coronavirus, Dengue fever, HPV, herpes simplex, Varicella-Zoster virus) and their long-term effects (e.g. long Covid), people who have completed cancer treatment and anyone whose immunity is weakened or disturbed
Lactose free, gluten free, GMO free, hormone free and 100% vegan.
Each capsule contains 500mg of AHCC, 20mg of vitamin C, 3.5mg of zinc and 27.5μg of Selenium
Purchase options:
- 60 capsules - £42 (70p per capsule)
- 180 capsules - £117 (65p per capsule)
Please refer to the 'Directions for use' section below to decide how much you need.
Price range: £42.00 through £117.00 (VAT included)
FREE delivery for purchases over £60
Secured payment
Frequently asked questions
AHCC®+3 is for people who need to improve their immunity.
It is especially designed for:
- people people fighting infections (e.g. flu, respiratory syncytial virus, coronavirus, Dengue fever, HPV, herpes simplex, Varicella-Zoster virus) and their long-term effects (e.g. long Covid),
- people who have completed cancer treatment and are reconditioning their immune system, and
- people such as the elderly and immunocompromised whose immunity needs support to maintain general health.
In infectious disease, AHCC® supplements are used to:
- prevent, manage and clear infections (e.g., flu, respiratory syncytial virus, coronavirus, Dengue fever, HPV, herpes simplex, Varicella-Zoster virus)
- help manage long-term effects of infections (e.g., 'long' Covid)
In cancer, alongside their standard treatments, patients use AHCC® to:
- reduce the side effects of chemotherapy and radiotherapy after the therapy,
- improve quality of life, and
- potentially lengthen postoperative survival (currently clinical evidence supports use for hepatocarcinoma, the most common form of liver cancer, only).
Healthy people use AHCC® for general health maintenance, immune support and to enhance their energy, especially when they feel that their health is challenged.
AHCC® stands for active hexose correlated compound. It is an immune support dietary supplement produced from the root-like filaments (mycelia), of shiitake mushroom (Lentinula edodes).
It is produced by AminoUp, a Japanese biochemical manufacturer, by culturing mycelia, extracting and concentrating the active ingredient, AHCC®.
AminoUp originally developed AHCC® in 1987 in collaboration with the University of Tokyo. AHCC® has since been used in more than 40 countries for over 30 years, with accumulated scientific evidence in cancer and infectious diseases.
AHCC® is rich in a powerful immune modulator known as partially-acylated α-1,4 glucans.
AHCC® helps strengthen the immune system by supporting the body’s immediate and long-term defences:
- It activates the cells and proteins which play a key part in fighting the early stages of infection (innate immune response).
- At the same time, it stimulates the body’s immune response to specific viruses or bacteria (adaptive immune response). This helps your body ‘remember’ the germs that have attacked it before and quickly respond, providing lasting protection and recovery.
- It acts as a prebiotic, nourishing ‘good’ bacteria in the gut microbiome. Since most of our immune cells live in the intestine, this improved gut environment directly supports a stronger immune response.
- It helps balance the body’s inflammatory response, which brings extra blood supply, immune cells and proteins to the site of an injury or germ ‘attack’. It promotes the production of proteins to support white blood cells while also reducing the levels of proteins involved in an excessive inflammatory response, which can be harmful. It helps ‘prime’ immune cells to stay alert to threats to the body, without triggering an overreaction, and in this way it promotes a more resilient and efficient immune system overall.
Selenium, zinc, and vitamin C work individually and together to keep the immune system strong.
Selenium supports powerful antioxidant enzymes. It helps activate immune cells like natural killer cells, which are part of your body’s immediate response, and T cells, which have learnt to recognise and attack harmful germs. It also works to balance the body’s inflammatory response. Its benefits are most noticeable in people who are low in selenium or under stress.
Zinc is vital for building and activating immune cells, keeping the skin and gut lining healthy, and helping the body control inflammation. Taking zinc can shorten the length of infections, improve recovery, and boost the effect of vaccines, especially in older adults.
Vitamin C strengthens the body’s barriers against germs, helps white blood cells find and destroy pathogens, and protects immune cells from damage. Regular vitamin C intake can reduce the duration and severity of colds and infections, while supporting healing and overall immune resilience.

Yes you can.
For women with HPV, Papilocare Vaginal Gel is recommended as the first-line treatment. Clinical studies have shown that using the gel for 6 months (daily for the first 3 months, then every other day for the next 3 months) can help support viral clearance.
If you want to complement this with an oral supplement, Papilocare ImmunoCAPS is specifically designed to enhance immune response against HPV in women living with the virus.
AHCC®+3 works differently from ImmunoCAPS. It provides broad support across multiple components of the immune system, and the two can be used together for greater support.
You can take AHCC®+3 concurrently with ImmunoCAPS if you want a stronger immune-support effect, or you may choose to switch to AHCC®+3 after completing a course of ImmunoCAPS to help maintain your general immune health.
Most human trials for AHCC® use 1-3g / day taken orally for up to 12 months. AHCC® has been generally safe. Reported adverse events are infrequent and usually mild, most commonly: temporary gastrointestinal upset (nausea, bloating, diarrhoea), occasional headache, or mild skin reactions. Serious adverse events directly attributable to AHCC® are rare or not reported in the clinical literature.
Since AHCC® modulates immunity, caution is advised for people with auto-immune diseases or people who are using immunosuppressants. Consult specialist clinicians.
Zinc, selenium and vitamin C are safe and well-tolerated in the recommended dosing range.
Not tested for use during pregnancy or breast feeding. Not suitable for people with autoimmune disease such as asthma, atopic dermatitis and IBD.
Following conditions require specialist advice before taking AHCC®: pregnancy/breastfeeding, severe immunosuppression, organ transplant recipients, known mushroom allergy, patients with autoimmune disease, user of immunosuppressants
Not suitable for children under 18 years old.
AHCC® is generally considered safe to take alongside most medications. However, it is known to induce certain enzymes in the cytochrome P450 (CYP450) family, including CYP2D6, CYP2C9, and CYP3A4, which play a key role in metabolising many drugs. Because of this, AHCC® may reduce the effectiveness of medications that rely on these enzymes.
If you are currently taking any of the following medications, it is important to consult your healthcare provider before starting AHCC®:
- HIV: Protease inhibitors and non-nucleoside reverse transcriptase inhibitors (NNRTIs)
- HCV: Protease inhibitors
- Bacterial infections: Clarithromycin, erythromycin
- Fungal infections: Itraconazole, ketoconazole, voriconazole
- Cancer treatments: Tyrosine kinase inhibitors (TKIs), mTOR inhibitors, taxanes, tamoxifen
For individuals who have completed these treatments, the risk of a drug-AHCC® interaction is low.
The efficacy and safety of AHCC® has been proven in over 100 publications including more than 30 human clinical studies. Below are selected studies in humans:
- Smith et al., 2022 (Frontiers in Oncology) – In a Phase II randomized, double-blind, placebo-controlled trial of women with persistent high-risk HPV, 3 g/day AHCC® for six months led to significantly higher rates of HPV clearance compared with placebo, with no safety concerns.
🔗 Frontiers / PMC
- Roman et al., 2013 (Nutrition Journal) – In healthy adults receiving an influenza B vaccine, short-term 3 g/day AHCC® improved lymphocyte subset balance and antibody titers, suggesting enhanced vaccine responsiveness.
🔗 PubMed
- Walshe et al., 2010 (Human Immunology) – In an open-label study of 30 healthy volunteers taking 3 g/day AHCC® for two months, there was a significant increase in CD4⁺ T cells producing IFN-γ and TNF-α, showing enhanced cellular immunity.
🔗 PubMed
- Madolangan et al., 2021 (Teikyo Medical Journal) – In tuberculosis patients living with HIV, 3 g/day AHCC® for six months improved clinical recovery and immune parameters compared to placebo.
🔗 Teikyo Medical Journal
Pre-clinical evidence
The efficacy of AHCC® has been proven in numerous animal and cell studies with a wide range of viruses, bacteria, fungi and parasites. Below are selected preclinical studies:
- Human papillomavirus (HPV): In mouse models of HPV16/18 infection, AHCC® reduced viral persistence and restored antiviral balance by increasing IFN-β, IFN-γ, and dendritic-cell activation—forming the mechanistic basis for later human HPV clearance trials. (Cell and animal studies)
- Influenza A/B: In murine influenza models, AHCC® decreased mortality and viral load while enhancing NK-cell cytotoxicity, interferon responses, and vaccine-induced antibody titers. (Multiple animal + human vaccine-response studies)
- SARS-CoV-2 (COVID-19): In vitro and mouse studies showed reduced viral replication, stronger type-I interferon signaling, elevated NK and CD8⁺ T-cell activity, and lowered inflammatory cytokines, suggesting potential antiviral and immunomodulatory effects. (Japan, 2021–2023)
- Herpes simplex virus (HSV-1/2): AHCC® reduced lesion size and viral load while enhancing NK- and T-cell responses in murine HSV infection. (Animal study)
- Hepatitis C virus (HCV): In Huh7 hepatocyte cultures, AHCC® suppressed viral replication and enhanced interferon-stimulated gene expression. (Cell culture studies)
- Listeria monocytogenes: In systemic bacterial infection, AHCC® lowered liver and spleen bacterial burden by activating macrophages and NK cells. (Animal study)
- Pseudomonas aeruginosa: Treated mice exhibited reduced lung bacterial counts, improved survival, and enhanced cytokine-driven clearance. (Animal model)
- Candida albicans: In murine candidiasis, AHCC® decreased fungal load and promoted macrophage phagocytosis with stronger Th1 cytokine responses. (Animal study)
- Mycobacterium tuberculosis: Both mouse TB models and small human HIV/TB trials reported reduced bacterial load, improved clinical scores, and elevated Th1/IFN-γ responses. (Preclinical + limited human data)
Across multiple infection models, AHCC® enhances innate immune defences (activating NK cells, macrophages, and dendritic cells) and promotes key antiviral cytokines such as IFN-α/β, IFN-γ, IL-12, and IL-2. It helps balance Th1/Th2 immune responses, reducing chronic inflammation and immune fatigue, leading to lower viral or bacterial loads, better survival outcomes, and stronger vaccine-induced antibody and T-cell responses.
Human clinical evidence
Human clinical evidence for AHCC® in cancer is promising but limited. The clearest signals are for the most common form of primary liver cancer, hepatocellular carcinoma. Small cohort and prospective reports suggest improved prognosis and longer survival.
There is also promising evidence for supporting patients during chemotherapy (especially for breast and ovarian cancer). Several small human studies report reduced chemotherapy-related neutropenia (abnormally low white blood cells called neutrophils), improved ability to tolerate treatment and some quality-of-life benefits. Most other positive data are immunologic markers (NK/T-cell activity).
Key points and sources
- Hepatocellular carcinoma (HCC): Small prospective/retrospective human studies report signals of longer survival and delayed clinical decline with AHCC use after diagnosis or resection. These studies are suggestive but not definitive. PubMed+1
- Breast cancer (chemotherapy support): Retrospective and small randomised trials report fewer neutropenia-related events and reduced need for stimulation of white blood cell production (G-CSF) in patients who took AHCC during chemotherapy. PMC+1
- Ovarian / gynaecological cancers: Pilot studies and small trials have tested AHCC® alongside chemotherapy for immune support and quality-of-life; results are mixed and sample sizes small. Larger feasibility/randomized trials are registered/underway. PMC+1
- Immune biomarkers: Multiple human studies show AHCC® raises NK activity, certain cytokines, and other immune markers in cancer patients — mechanistic evidence that might explain clinical signals.
Preclinical evidence for cancer
Preclinical evidence shows AHCC® as a broad immunomodulatory add-on therapy (adjuvant) (with repeated demonstrations of enhanced NK/CD8 activity, favourable cytokine shifts (IFN-γ/IL-12), tumour growth delay, and synergy with chemo/immunotherapy in multiple mouse models and in vitro cancer cell lines.
- Innate immune activation: AHCC® increases NK-cell activity, macrophage function and dendritic-cell maturation in mice and ex vivo human cells. These changes are accompanied by rises in IFN-γ, IL-12, and other Th1-type signals, which drive early antitumour responses and improved recognition/killing of tumour cells in vivo.
- Adaptive immune effects: In several mouse tumour models AHCC® promotes CD8⁺ cytotoxic T-cell responses and supports CD4⁺ helper activity (e.g., increased IL-2 and IFN-γ production). Some studies report reduced regulatory T cells (Tregs) or myeloid-derived suppressor cells (MDSCs), shifting the balance toward an immune-active tumour microenvironment.
- Direct tumour effects and combination with other therapies
- In vitro, AHCC® has been reported to increase apoptosis or sensitize cancer cell lines (breast, colon, melanoma, hepatoma) to cytotoxic agents.
- In vivo, AHCC® reduced tumour growth and metastatic spread in multiple mouse models (melanoma, colon, lung, hepatocellular carcinoma, pancreatic models).
- AHCC® frequently shows synergy with chemotherapy or immunotherapy in animals
- Some studies also report improved mitochondrial function (energy generation) and reduced oxidative stress in host immune cells, supporting sustained antitumour activity.
Supplementation in deficient or stressed populations enhances immune responsiveness, reduces infection severity, and supports vaccine efficacy. However, the benefits level out in people whose selenium levels are high enough.
Zinc supplementation reduces the duration of infection (especially respiratory), improves vaccine response in the elderly, and strengthens immune recovery in those who are deficient in zinc.
Clinically, vitamin C shortens the duration and reduces the severity of respiratory infections, supports wound healing, and improves immune resilience in stress, aging, or chronic illness.
We also recommend
Papilocare® External Genital Gel
 Shop Now
Shop Now
Papilocare® Immunocaps
 Shop Now
Shop Now
Papilocare® Vaginal Gel
 Shop Now
Shop Now
Papilocare® Vaginal Gel
- 7 tubes - £21.70 (£3.10 per tube)
- 21 tubes - £55.45 (£2.64 per tube)
- 84 tubes - £211.80 (£2.52 per tube) (original 6 month course)
- 105 tubes - £262.25 (£2.50 per tube) (the enhanced 6 month course)
Price range: £21.70 through £262.25 (VAT included)
 Shop Now
Shop NowPapilocare® Immunocaps
- 30 capsules - £34.30 (£1.14 per capsule)
- 90 capsules - £99.90 (£1.11 per capsule)
- 180 capsules - £195.20 (£1.08 per capsule)
Price range: £34.30 through £195.20 (VAT included)
 Shop Now
Shop NowOvosicare® Fertility
- 60 capsules - £39.90 (67p per capsule)
- 180 capsules - £114.10 (£0.63 per capsule)
Price range: £39.90 through £114.10 (VAT included)
 Shop Now
Shop NowSocial
Sign up to receive our newsletter and a welcome discount code:
Disclaimer: Information on this website is provided for informational purposes only and not intended as a substitute for the advice provided by your physician or other healthcare professional. You should not use the information on this website for diagnosing or treating a health problem or disease, or prescribing any medication or other treatment. For medical advice, diagnosis and prescription, please consult a healthcare professional. More Information >
Disclaimer: Information on this website is provided for informational purposes only and not intended as a substitute for the advice provided by your physician or other healthcare professional. You should not use the information on this website for diagnosing or treating a health problem or disease, or prescribing any medication or other treatment. For medical advice, diagnosis and prescription, please consult a healthcare professional.
© LivBio Limited 2024 All Rights Reserved.